CLINICAL TRIAL RESULTS
In two clinical studies,‡
XOLAIR was shown to help reduce the severity of itch* and the number of hives†§
§In patients who continued to have symptoms that were not controlled by H1 antihistamine treatment. Individual results may vary.
Results of a CSU study in adults and adolescents||
XOLAIR was studied for 24 weeks to measure the severity of itch* and number of hives† (on a weekly basis) of patients with chronic hives who continued to have hives while on antihistamine therapy. Individual results may vary.
Study design
A total of 319 people with chronic hives who were already taking an antihistamine participated in the study.

Primary goal
The primary goal of the study was to assess the change from baseline weekly itch severity score at Week 12.
Secondary goals
The secondary goals of the study included the change from baseline hive count score at Week 12 and the proportion of complete responders (no itch or hives) at Week 12 among other measures.
*To measure itch severity, patients were asked to write down how itchy they felt every morning and night on a scale of 0-3. The average daily score was then added together over 7 days for the weekly itch severity score.
†To measure the hive score, patients were asked to count the number of hives on their bodies twice daily (0=no hives, 1=1 to 6 hives, 2=7 to 12 hives, and 3=more than 12 hives). The average daily hive score was then added together over 7 days for the weekly hive count score.
‡Based on results from CSU Studies 1 and 2.
‖Based on results from CSU Study 1, a 24-week clinical study.
¶77 patients were given 75 mg of XOLAIR. The 75 mg dose did not demonstrate consistent evidence of efficacy and is not approved for use.
#A placebo does not contain any active medicine.
Study 1 results in adults and adolescents
Itch severity reduction
Itch severity* reduction at 12 weeks
300 mg, n=81

150 mg, n=80

Placebo, n=80

Relief observed as early as 2 weeks
A post hoc analysis is a review of clinical trial data that was not planned before data collection was started and it is not designed to prove cause and effect. Therefore, conclusions should not be drawn based on this information.
300 mg, n=81
54%
reduction
150 mg, n=80
36%
reduction
Placebo, n=80
25%
reduction
Hive count score
Hive count score† reduction at 12 weeks
300 mg, n=81

150 mg, n=80

Placebo, n=80

Complete relief
Complete relief is possible at 12 weeks
300 mg, n=81

More than 1 in 3
patients (36%) were completely itch- and hive-free.
150 mg, n=80

Nearly 1 in 6
patients (15%) were completely itch- and hive-free.
Placebo, n=80

Around 1 in 10
patients (~10%) were completely itch- and hive-free.
Visualize potential reduction in hives symptoms at 12 weeks
Possible level of hive reduction after 12 weeks on XOLAIR*
- At 300 mg, patients' weekly hive count score† was reduced by over 67%
- At 150 mg, patients' weekly hive count score† was reduced by 50%
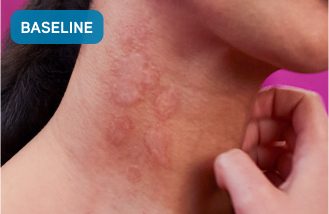
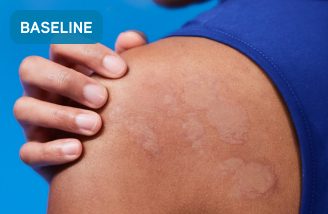
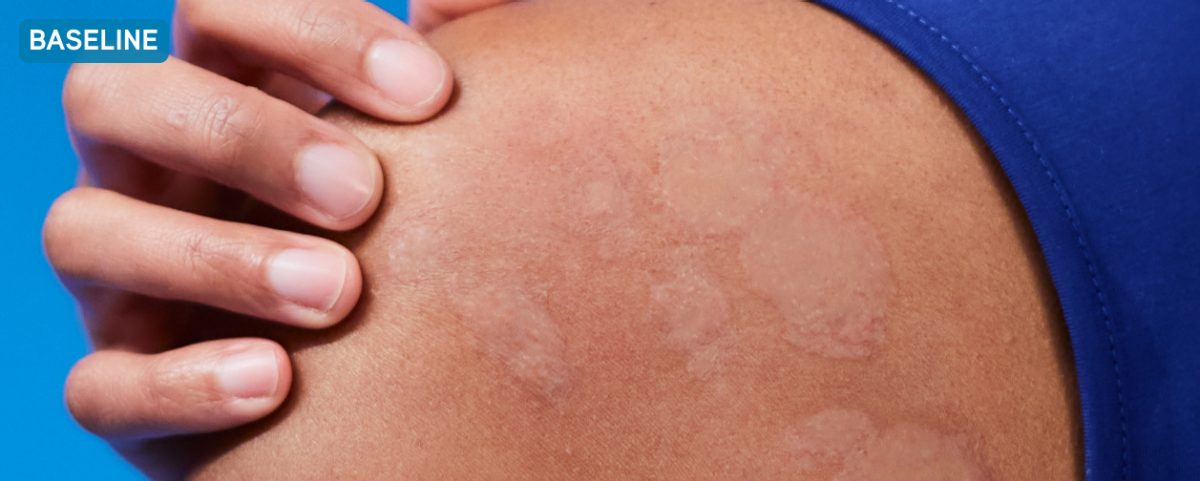
Before XOLAIR
Typical chronic hives symptoms before treatment with XOLAIR
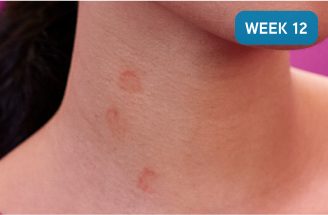
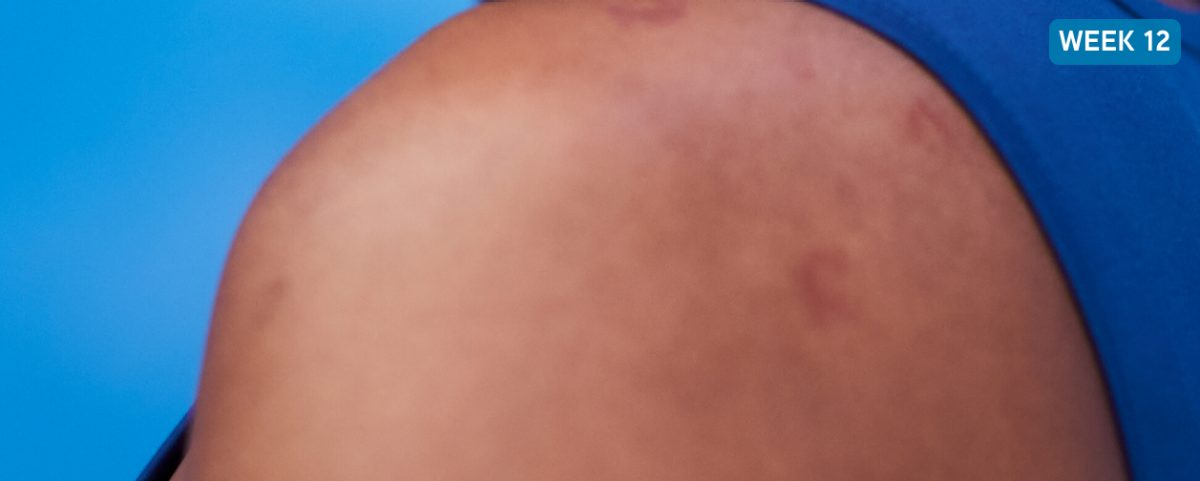
After XOLAIR
Shows how a 67% reduction of the weekly hive count score† can look after 12 weeks on XOLAIR (300 mg)
Not a real patient. Images are for illustrative purposes only. Individual results may vary.
Itch and hives resolved completely in more than 1 in 3 patients on 300 mg after 12 weeks on XOLAIR*
- At 300 mg, more than 36% of patients were completely itch‡ and hive†-free
- At 150 mg, almost 15% of patients were completely itch‡ and hive†-free
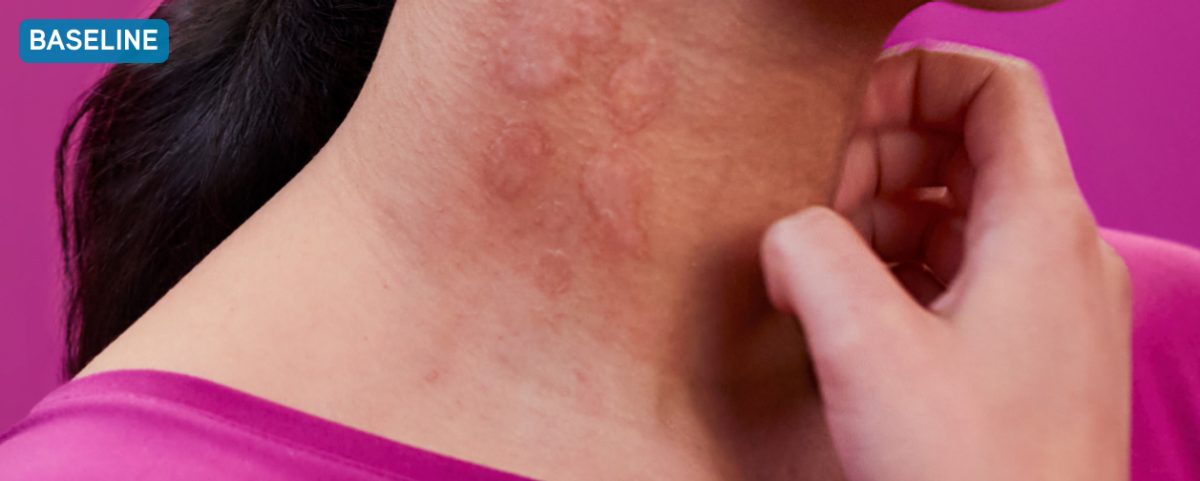
Before XOLAIR
Typical chronic hives symptoms before treatment with XOLAIR
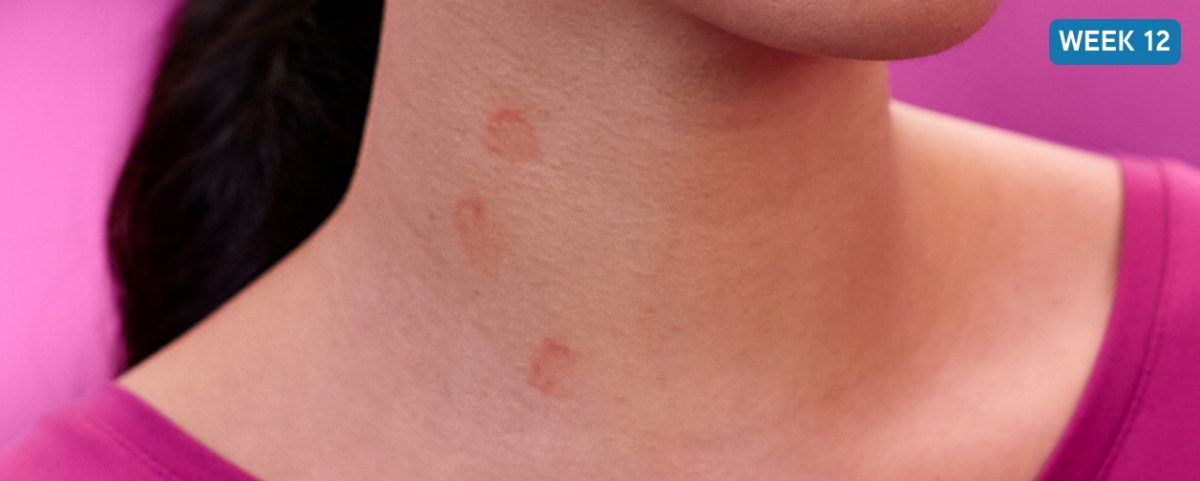
After XOLAIR
Possible complete response after 12 weeks on XOLAIR (300 mg)
Not a real patient. Images are for illustrative purposes only. Individual results may vary.
These examples of improvement are based on results from Chronic Hives Study 1, a 24-week study.
Similar results were observed in a second study‡
XOLAIR was studied for a second time for 12 weeks to measure the severity of itch* and number of hives† (on a weekly basis) of CSU patients who continued to have hives while on antihistamine therapy. The study involved 322 patients already taking an antihistamine and was split into 3 groups (79 were given 300 mg of XOLAIR, 82 were given 150 mg of XOLAIR, 79 were given a placebo).
Itch severity and hive count scores
Itch severity* reduction at 12 weeks
300 mg, n=79

150 mg, n=82

Placebo, n=79

Hive count score† reduction at 12 weeks
300 mg, n=79

150 mg, n=82

Placebo, n=79

Complete relief
Complete relief is possible at 12 weeks
300 mg, n=81

More than 4 in 10
patients (44%) were completely itch- and hive-free.
150 mg, n=80

More than 1 in 5
patients (22%) were completely itch- and hive-free.
Placebo, n=80

1 in 20
patients (5%) were completely itch- and hive-free.
‡82 patients were given 75 mg of XOLAIR. The 75 mg dose did not demonstrate consistent evidence of efficacy and is not approved for use.
To ask questions or obtain more information about XOLAIR study results, please consult your doctor.
What are clinical studies?
To learn how XOLAIR might help patients with chronic hives, medical teams conducted research—this is known as clinical studies. Experts wanted to know how much XOLAIR could reduce (1) the severity of itch and (2) the number of hives.
Not all clinical studies are the same. However, many factors, such as the number of people who participate, types of people, the location of the participants, and other external or internal matters, can play a role in the outcome of a clinical study. This is why they are so important to do—and why more than one study may be done. Also remember that all people are unique—even if they do share a common condition such as chronic hives.
Two studies were conducted looking at the effect of XOLAIR in patients with chronic hives. In the studies, all the patients remained on an H1 antihistamine. Patients were then split into 3 different groups. Two groups (the XOLAIR groups) received different doses of XOLAIR, and the third (the control group) received a placebo.
What are control groups?
In this group, patients received H1 antihistamine treatment and a placebo. A placebo is a look-alike treatment with no active medicine that can affect an illness. Placebos can take many forms, such as a pill, an injection, or even a procedure. In this case, it was an injection.

Talk to your allergist about XOLAIR
XOLAIR has been prescribed to over 380,000 CSU patients in the US since 2014.* If you have hives that last for 6 weeks or more, download our patient journey guide to help you start the conversation to see if XOLAIR could be right for you.
*Data on file (December 2024). Genentech USA, Inc. South San Francisco, CA.
You’ve got a whole support network on your side
From diagnosis to treatment, everyone’s CSU experience is unique. The Support For You program is designed with YOU in mind. Access free 1-on-1 sessions and learn about programs that may help you save on XOLAIR.